Please select the object link below for more information on how to configure the object.
Object |
Shared with other SmartSolve© Applications |
Required for SmartComplaintsTM |
|
Yes |
Yes |
|
|
No |
Yes |
|
|
No |
No |
|
|
No |
No |
Please see Numbering Scheme under System Wide Setup.
The default submission status can be changed from INWORKS to anything needed by the users. The following default statuses are available in the system:
Status |
Submission Type |
Description |
|
INWORKS |
Manual | eSubmission |
This status displays next to any regulatory report that must be submitted as a result of the decision tree conclusion.
|
|
MANUAL |
Manual |
This status displays if any regulatory report was submitted manually.
|
|
PENDING |
eSubmission |
This status displays next to any regulatory report that was submitted to the regulatory agency and is awaiting regulatory approval.
|
|
FAILED |
eSubmission |
This status displays next to any regulatory report submitted to the regulatory agency and has failed any of the three acknowledgements.
|
|
PASSED |
eSubmission |
This status displays next to any regulatory report submitted to the regulatory agency and has passed the third Acknowledgement. This means that the eSubmission is now in the MAUDE database (only for eMDR).
|
With the introduction of the new submission status, it may be necessary for customers to set a different default than INWORKS when a report is required, based on the policy results in a decision tree. For example, if there are periodic reports that are necessary to be completed within a certain time frame, but are not dependent on the closure of the complaint record, then customers may want to change the status to something else, for example, PERIODIC. This will also enable customers to search this status over a period of time to find all the incidents that need to be included within their reports.
A tag can be added to any report defined in the FormsConfig.xml file so that when the decision tree is run, and that specific report is required, the status of that report will display as the default that is defined for the report.
|
NOTE: This does not apply to follow-up reports. It applies only to the initial report when the decision tree is run. |
For example the user might add the following tag to a report that is defined as a quarterly submission report or a Periodic report. The following tag can be placed anywhere within the structure for a specific report.
DefaultSubmissionStatus="Periodic"
1. From the SmartSolve\SubmissionForms folder, open the FormsConfig.XML file.
2. Add the tag DefaultSubmissionStatus="Anything" anywhere for this report within the valid tag structure.
3. Where Anything = whatever is required by regulatory personnel. This is the value displayed instead of INWORKS when the forms are initially created.
4. Save the file.
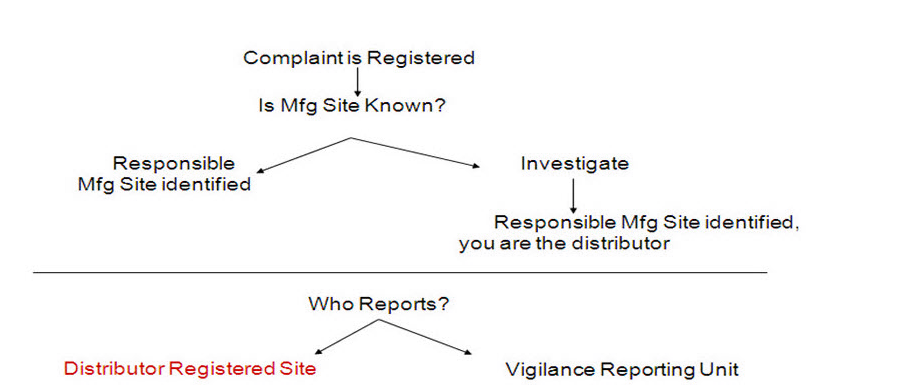
Regulatory Reporting policies should be created for regulated companies who are required to report an adverse event report and have one or more of the following:
Multiple Manufacturing or specification development sites
Distributors and importers
Reporting units
This type of policy provides the ability to identify the appropriate pre-defined numbering scheme and to generate the Manufacturer Report #on the MedWatch 3500A form based on which site is responsible for manufacturing or specification development of the product. The policy records information (which includes reporting site details) and is transferred to the Submission folder of the complaint after the decision tree is run.
|
NOTE: If a policy is not defined, the SmartComplaintsTM default numbering scheme for submission numbering is used for the submission form. Please see Numbering Schemes. |

Once the numbering schemes are attached to the policies, they will transfer to the regulatory reports that are loaded from the complaint record.
The following rights must be assigned to a user to manage or view Regulatory Reporting Policies:
Rights |
Description |
|
CSP_MANAGE |
Allows administrator to add new Regulatory Reporting Policies, modify existing Policy, or delete Policies.
|
|
CSP_VIEW |
Allows user to access Regulatory Reporting Policy to View.
|
Please see Rights Group to assign Rights Groups to users
· Global Regulatory Reporting Policies will be inherited by all local organizations.
· Local Regulatory Reporting Policies will only be viewed and used within that local organization.
To write a regulatory reporting policy, you must be familiar with writing a test condition in SQL using the un-translated field names of any of the exception template entries driving the policy. These SQL statements are not checked; therefore, human intervention is required.
The following are lists of translated and untranslated fields for the regulatory reporting policy:
Translated Field |
Untranslated Field |
|
Manufacturing Site |
CEX_MFCTR_SITE
|
|
Importer/Distributor Site |
CEX_IMPRT_DIST
|
1. From the main menu select Admin > Policy > Exception/Issue Related > Regulatory Reporting Policy.
2. Right click and select Add then select the Regulatory Body.
3. Enter information in the following fields:
Field |
Description |
|
Regulatory Body |
Select a regulatory body from the drop down list.
|
|
Test Sequence |
Enter Test Sequence (system evaluates from lowest to highest sequence to see which sequence first meets this requirement).
|
|
Test Condition |
Enter the Test Condition using the un-translated field name(s).
|
|
Test Description |
Enter Test Description which will better explain what the Test Condition means in laymen’s terms.
|
|
Reporting By |
Select the Reporting By site. Manufacturing Site - The registered establishment (facility mfg/design) will report their MedWatch Report and will supply the contact. Configure the following fields: Manufacturing Numbering Scheme Manufacturing Site Registration Number Manufacturing Site Contact Reporting Site - Typically the company reporting group/division that submits the MedWatch reports for the products that they are responsible to maintain. Configure the following fields: Reporting Site Reporting Site Contact The manufacturing site’s contact information that will populate into Section G of the MedWatch report for the FDA to contact if they have questions. Importer/Distribution Site - The registered establishment (importer) that imports or distributes products that they do not manufacture or repackage. They will submit the Medwatch to the FDA and the manufacturer. Configure the following fields: Importer/Distributor Numbering Scheme Importer/Distributor Site Contact
|
|
Manufacturing Numbering Scheme |
Zoom to select a numbering scheme. |
|
Manufacturing Site Registration Number |
Enter a registration number, if applicable. |
|
Manufacturing Site Contact |
Zoom to select a contact for the policy. |
|
Reporting Site |
Zoom to select a reporting site.
|
|
Reporting Site Contact |
Zoom to select a reporting site contact, if applicable. |
|
eSubmission Enabled |
Check the eSubmission checkbox if eSubmissions will be used with this policy.
|
|
|
NOTE: this functionality turns on the ability to use the html template for data entry of a requlatory form which will create an xml file for submission. Please see eMDR Reporting Setup and eMDV Reporting Setup
|
|
Importer/Distributor Numbering Scheme |
Zoom to select a numbering scheme, if applicable. |
|
eSubmission File Naming Format |
Enter the eSubmission File Naming Format if eSubmissions will be used with this policy.
|
|
|
NOTE: The default naming convention is defined in the formconfig.xml file, if a unique name needs to be defined at the policy level, this allows the actual name of the xml file that is output to be configured. Please see eMDR Reporting Setup and eMDV Reporting Setup.
|
|
Importer Distributor Contact |
Zoom to select a distributor contact, if applicable. |
|
eSubmission Folder Path |
Enter the eSubmission Folder Path if eSubmissions will be used with this policy.
|
|
|
NOTE: the eSubmission Folder Path can be defined in the FormsConfig.xml file as a default path and then specific folder paths can be defined here for either plant specific or product type output. For Example: \\corpresearch\apps\RegulatoryAffairs\FDASubmissionFolder Please see eMDR Reporting Setup |
|
Add Another |
Check this option if you wish to add another Regulatory Policy at this time.
|
4. Click the Save button.
Regulatory reporting policies can be edited, deleted and managed accordingly by an administrator.
1. From the SmartSolve© menu select Admin > Policy >Exception/Issue Related > Regulatory Reporting Policy.
The Regulatory Reporting Policies list displays.
2. Click the ![]() icon to switch to Local view (if applicable).
icon to switch to Local view (if applicable).
3. Select the check box of the Policy to edit, then select Action > Edit from the main menu (or right click).
4. Edit any information for your policy.
5. Click the Save button.
All changes should now be reflected in the list.
1. From the SmartSolve© menu select Admin > Policy | Exception/Issue Related | Regulatory Reporting Policy.
The Regulatory Reporting Policies list displays.
2. Click the ![]() icon to switch to Local view (if applicable).
icon to switch to Local view (if applicable).
3. Select the check box of the policy to delete, then select Action >Delete from the main menu (or right click).
4. Click the Save button.
The policy has now been removed from the system.
The PDF="3500aForm.pdf " file is the Pilgrim provided, FDA approved, MedWatch template used to generate the PDF form. If the 3500a needs to be approved or changed this is the file that needs to be changed. Any changes made to this form need to be approved by the FDA.
Application Name\SubmissionForm\3500aForm.pdf
Significance of Mapped Fields
The complaint record fields described in the tables below map automatically to fields in the regulatory submission forms. All other fields on the submission forms must be entered manually.
Some mapped fields in the Complaint record are read-only in the form. Other mapped fields can be edited in the form. However, if the user edits the regulatory submission form itself without changing the same information in the Complaint record and then recreates the form, the edits in the form will be overwritten by the unchanged complaint record output.
For a follow-up form, it is possible to exclude fields that appeared in the original form. For further technical advice on excluding fields from follow-up forms, consult Pilgrim Professional Services.
MedWatch Form Page 1
Section |
Field Title |
Data Source |
Field/Value/Comments |
|
Header |
Company Name |
SubmissionForms\3500aForm.xsl |
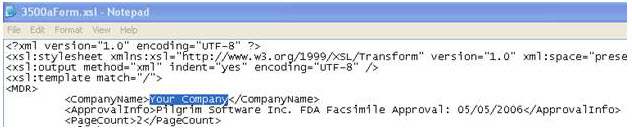
To be replaced by customer Current Value: "Your Company" should appear at the top center of each page of each form. |
|
|
FDA facsimileMfr Report # approval |
SubmissionForms\3500aForm.xsl |
"Pilgrim Software Inc. FDA Facsimile Approval: 05/05/2006" should appear top right of the page on second line |
|
|
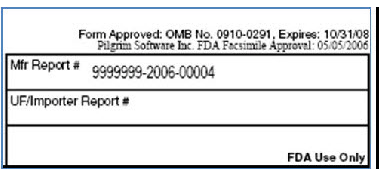
Mfr Report # |
Complaint/V_BAH_BASE_ ATTACH_CXP/BAH_REPORTNUMBER |
This will be auto generated on Submit using the Numbering Scheme defined as follows: a) If Submission Reporting Site = "Manufacturing Site" or "Reporting Site" and the Manufacturing Numbering Scheme is defined. b) If the Manufacturing Numbering Scheme is NOT defined, the system will assign the number from the Numbering Scheme defined for the corresponding form type in the Formconfig.xml. This value is populated only for "30-day MDR" and "5-day MDR/" Format 9999999-YYYY-XXXXX 9999999 – Hard coded value by customer (your Manufacturing Registration Number) YYYY - Replaced by current year XXXXX – Actual report number increment (reset every year).
|
|
|
|
|
NOTE: If this value is populated, "UF/Dist report#" should not be populated.
|
|
|
UF/Dist report# |
Complaint/V_BAH_BASE_ATTACH_ CXP/BAH_REPORTNUMBER |
This will be auto generated on Submit using the Numbering Scheme defined as follows: a) If Submission Reporting Site = "Importer/Distributor Site" and the Importer/Distributor Numbering Scheme is defined. b) If the If importer/Distributor Numbering Scheme is NOT defined, the system will assign the number from the Numbering Scheme defined for the corresponding form type in the Formconfig.xml. This value is populated only for "30-day MDR Distributor." Format 9999999-YYYY-XXXXX 9999999 – Hard coded value by customer (your Distributor Registration Number) YYYY - Replaced by current year XXXXX – Actual Report number increment (reset every year) |
|
|
|
|
NOTE: The value will support a UF 9999999999-yyyy-xxxxx value. The field will be an editable field to be manually entered before submission.
|
|
B |
3. Date of event (mm/dd/yyyy) |
QXP_OCCURENCE_DATE |
Occurrence Date |
|
|
4. Date of This Report (mm/dd/yyyy) |
QXP_REPORT_DATE |
Report Date |
|
|
5. Describe event or problem |
QXP_SHORT_DESC + QXP_ DESCRIPTION |
This field should auto fill with the "Short Description" and/or "Description" from the Complaint Description section of the Exception form |
|
D |
1. Brand name |
CXP_BRAND_NAME |
Brand Name from product information section |
|
|
4. Model # |
PXP_PRODUCT_NAME |
Part Name from the product Information section |
|
|
4. Catalog # |
CXP_CATALOG_NUMBER |
Catalog Number from the product Information section |
|
|
4. Expiration Date (mm/dd/yyyy) |
CXP_EXP_DATE |
Expiration Date from the product Information section |
|
|
3. Manufacturer name & address |
Complaint/MANUFACTURER /ORU_NAME Complaint/MANUFACTURER_ ADDRESS/PHY_CITY Complaint/MANUFACTURER _ADDRESS/PHY_STATE |
Manufacture Name and Address for the manufacture site defined in the Additional folder |
|
|
D. Suspect medical device 9. Device available for evaluation? |
CEX_SMP_AVAILABLE |
"Is Sample Available? from the Additional section Possible Values : Yes/No |
|
|
D. Suspect medical device 9. returned to manufacturer on (mm/dd/yyyy) |
CEX_SMP_RECEIPT_DATE |
Check the box and Sample Receipt Date in Additional Section |
|
E |
E. Initial reporter 1. Name, address & phone# |
CMC_NAME CMC_STREET CMC_CITY, CMC_STATE CMC_ZIPCODE CMC_COUNTRY |
Contact Name Street City, State Zip Country Fax: Fax No Email: Email_address From the Contact folder for Contact Type = "CUSTOMER" (First record if there are two contacts with the type "CUSTOMER.") |
|
|
Phone # |
CMC_PHONE |
Phone From the Contact folder for Contact Type = "CUSTOMER" (First record if there are two contacts with the type "CUSTOMER.") |
|
|
E. Initial reporter 3. Occupation |
CMC_TITLE |
Title From the Contact folder for Contact Type = "CUSTOMER" (First record if there are two contacts with the type "CUSTOMER.") |
MedWatch Form Page 2
Section |
Field Title |
Data Source |
Field/Value/Comments |
|
F |
6. Date User Facility or Importer became Aware of Event (mm/dd/yyyy) |
CXP_AWARE_DATE |
Incident Aware Date from the Customer Information section |
|
|
8. Date of This Report (mm/dd/yyyy) |
QXP_REPORT_DATE |
Report Date |
|
|
2. UF/Importer Report Number |
Complaint/V_BAH_BASE_ATTACH_ CXP/BAH_REPORTNUMBER |
Same as UF/Dist report# under header section, and editable field for user to hand enter if required. |
|
|
1. User Facility / Importer check box |
|
Importer If Submission Reporting Site = "Importer/Distributor"
|
|
|
|
|
NOTE: User Facility Check box is not supported by system. It is user keyable.
|
|
|
3. User Facility or Importer Name and Address |
|
If Submission Reporting Site = "Importer/Distributor", then name and address of Importer/Distributor Site defined in the Additional folder. If Submission Reporting Site = "Reporting Site" then Name and address of Reporting Site defined in the Reporting Default. |
|
|
4. Contact Person |
|
Primary Contact of the Site defined in F3 |
|
|
5. Phone Number |
|
Phone Number of the Primary Contact in F3 |
|
|
7. Type of Report |
|
Auto-fill if this is an initial or follow-up report If initial, check Initial box. If follow-up: check Follow-up box and enter follow-up sequence number. |
|
|
14. Manufacturer Name/Address |
|
Name and Address of the Manufacturing Site defined in the Additional folder. |
|
G |
G. All manufacturers 1. Contact office, name, address 2. Phone |
SubmissionForms\3500aForm.xsl |
|
|
|
G. All manufacturers 4. Date received by manufacturer (mm/dd/yyyy) |
CXP_AWARE_DATE |
This should auto fill with the Incident Aware Date under the Customer Information section. |
|
|
G. All manufacturers 7. Type of report |
BAH_CONCLSN_NAME And BAH_FOLLOWUP_NO |
Check box checked based on the Form type selected: 30 Day – if 30-day MDR 5-Day – if 5-Day MDR
|
|
|
9. Mfr report number |
Complaint/V_BAH_BASE_ATTACH_CXP /BAH_REPORTNUMBER |
Same as "Mfr Report #" under Header section |
|
H |
H. Device manufacturers only 10. Additional manufacturer narrative |
BTK_SIGNOFF_COMMENT |
Investigation Result From Resolution section: "Additional Manufacturer Narrative" - The check box will be checked. NOTE: This should auto-fill if the Results of Investigation task in the issue workflow is signed off |
EMDV Form
Section |
Field Title |
Data Source |
Field/Value/Comments |
|
P1 sec 2 |
b. Address |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: Your Street Your City, Your State 99999 Your Country |
|
|
c. Contact person Name |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: Your Contact Name |
|
|
e. Telefax Number |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: (XXX)XXX-XXXX |
|
|
d. Telephone Number |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: (XXX)XXX-XXXX |
|
|
f. Report date |
QXP_REPORT_DATE |
|
|
P2 sec 4 |
c) Model or Catalogue Number |
CXP_CATALOG_NUMBER |
Catalog Number from the Product Information section |
|
|
d) Serial number(s) or lot number(s) |
PXP_LOT_NUMBER |
|
|
|
Address |
CMC_STREET CMC_CITY, CMC_STATE CMC_ZIPCODE CMC_COUNTRY |
Street City, State Zip Country From the Contact folder for Contact Type = CUSTOMER |
|
|
j) Incident reported by (user or other source) |
CMC_NAME |
Contact Name From the Contact folder for Contact Type = CUSTOMER |
|
|
Telephone number |
CMC_PHONE |
CMC_PHONE Phone From the Contact folder for Contact Type = CUSTOMER |
|
|
Date reported |
QXP_REPORT_DATE |
|
|
|
k) Incident date |
QXP_OCCURENCE_DATE |
|
|
|
l) Incident description |
QXP_SHORT_DESC & QXP_DESCRIPTION |
This field should auto-fill with the "Short Description" and/or "Description" from the Complaint Description section of the Exception form. |
|
|
p3_4n_ManufacturesComments |
BTK_SIGNOFF_COMMENT |
Investigation Result from the Resolution section. |
|
|
|
|
NOTE: This should auto-fill if the Results of Investigation task in the issue workflow is signed off.
|
|
P4 sec 2 |
b. Address |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: Your Street Your City, Your State 99999 Your Country |
|
|
c. Contact Person Name |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: Your Contact Name |
|
|
d. Telephone Number |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: (XXX)XXX-XXXX |
|
|
e. Telefax Number |
SubmissionForms\ EU-QR-P12-01MedForm01.xsl |
To be replaced by customer. Current Value: (XXX)XXX-XXXX |
|
|
2.f) Date of this final report |
QXP_REPORT_DATE |
Catalog Number from the Product Information section. |
|
P5 sec 4 |
c) Model or Catalogue Number |
CXP_CATALOG_NUMBER |
|
|
|
d) Serial number(s) or lot number(s) |
PXP_LOT_NUMBER |
|
|
P6 sec 4 |
m) Result and conclusion of manufacturer's investigation |
BTK_SIGNOFF_COMMENT |
Investigation Result From the Resolution section. |
|
|
|
|
NOTE: This should auto fill if the Results of Investigation Task in the issue workflow is signed off.
|
Canada TPP Form
Section |
Field Title |
Data Source |
Field/Value/Comments |
|
General Information (P1) |
2. Name of Reporter |
QXP_REPORTER_NAME |
|
|
|
4. Institution/ Company |
CXP_CLIENT_NAME |
|
|
|
5. Address |
CMC_STREET, CMC_CITY, CMC_STATE, CMC_COUNTRY |
CMC_TYPE= CUSTOMER |
|
|
6. Postal Code |
CMC_ZIPCODE |
CMC_TYPE= CUSTOMER |
|
|
7. Telephone |
CMC_PHONE |
CMC_TYPE= CUSTOMER |
|
|
8. Fax |
CMC_FAX |
CMC_TYPE= CUSTOMER |
|
|
9. Contact Person (if different from reporter) |
CMC_NAME |
CMC_TYPE= CUSTOMER |
|
|
13. Is the device available for evaluation? |
CEX_SMP_AVAILABLE |
|
|
|
14. Date of Incident |
QXP_OCCURENCE_DATE |
|
|
|
15. Manufacturer/Importer Awareness Date: |
CXP_AWARE_DATE |
|
|
Medical Device Information |
16. Trade Name |
PXP_PRODUCT_NAME |
|
|
|
17. Manufacturer Medical Device Identifier: |
PXP_PRODUCT_CODE |
|
|
Problem Description (P2) |
41. Details of Incident Including Consequences to Patient, User or Other Person, and Description of Other Devices or Accessories Involved in the Incident |
QXP_SHORT_DESC & QXP_DESCRIPTION |
This field should auto-fill with the Short Description and/or Description from the Complaint Description section of the Exception form. |
|
|
42. Manufacturer's Preliminary Comments |
BTK_SIGNOFF_COMMENT |
Investigation Result From Resolution section. |
|
|
|
|
NOTE: This should auto-fill if the Results of Investigation task in the issue workflow is signed off.
|
Australia TGA Form
Section |
Field Title |
Data Source |
Field/Value/Comments |
|
Header |
-Mfr report # |
BAH_REPORTNUMBER |
This will be auto-generated using the "Australia Numbering" Numbering Scheme. Format: 9999999-YYYY-XXXXX 9999999 – Hard coded value by customer. (Your Manufacturing Registration Number) YYYY - Replaced by current year XXXXX – Actual Report number Increment (Need to reset Every Year) |
|
I- Administrative Information |
Date of this report (dd-mmm-yyyy |
QXP_REPORT_DATE |
|
|
|
Date of adverse event (dd-mmm-yyyy) |
QXP_OCCURENCE_DATE |
|
|
|
Date mfr aware (dd-mmm-yyyy |
CXP_AWARE_DATE |
|
|
Person (authorized representative) submitting this report |
Name |
CMC_NAME |
|
|
|
Company |
CMC_COMPANY |
|
|
|
Address |
CMC_STREET CMC_CITY, CMC_STATE, CMC_ZIPCODE CMC_COUNTRY |
|
|
|
Tel |
CMC_PHONE |
|
|
|
Fax |
CMC_FAX |
|
|
|
|
CMC_E_MAIL |
|
|
III- Healthcare Facility Information |
Name |
CXP_CLIENT_NAME |
|
|
|
Address |
CMC_STREET CMC_CITY, CMC_STATE, CMC_ZIPCODE CMC_COUNTRY |
|
|
|
Tel |
CMC_PHONE |
|
|
|
Fax |
CMC_FAX |
|
|
|
|
CMC_E_MAIL |
|
|
|
Contact name at site of event |
CMC_NAME |
|
|
IV- Device Information (Specific Device Information) |
Brand Name |
CXP_BRAND_NAME |
|
|
|
Model # |
PXP_PRODUCT_NAME |
|
|
|
Catalogue # |
CXP_CATALOG_NUMBER |
|
|
|
Ser. or Lot #'s |
PXP_LOT_NUMBER |
|
|
II- Clinical Event Information |
Description of event or problem |
QXP_SHORT_DESC & QXP_DESCRIPTION |
This field should auto-fill with the Short Description and/or Description from the Complaint Description section of the Exception form. |
|
V- Results of Mfr's Investigation |
Manufacturers Device Analysis Results (Specify, for this event, details of investigation methods, results, and conclusions) |
BTK_SIGNOFF_COMMENT |
Investigation Result From Resolution section |
|
|
|
|
NOTE: This should auto-fill if the Results of Investigation task in the issue workflow is signed off.
|
Japan ARR Form
Section |
Field Title |
Data Source |
Field/Value/Comments |
|
Appendix form 7 1. Control information |
3) Date problem occurred |
QXP_OCCURENCE_DATE |
|
|
|
5) Date reported |
QXP_REPORT_DATE |
|
|
|
4) Date information received |
CXP_AWARE_DATE |
|
|
3. Information on medical device |
1) Trade name of medical device |
PXP_PRODUCT_NAME |
|
|
|
3) Detailed information on medical device |
QXP_SHORT_DESC & QXP_DESCRIPTION |
|
|
4. Investigation results and countermeasures |
1) Investigation results |
BTK_SIGNOFF_COMMENT |
|
|
1. Control information |
Information received |
|
|
|
|
4) Date reported |
QXP_REPORT_DATE |
|
|
2. Information on medical device |
1) Trade name of medical device |
PXP_PRODUCT_NAME |
|
|
|
3) Detailed information on medical device |
QXP_SHORT_DESC & QXP_DESCRIPTION |
|
|
3. Investigation results and countermeasures |
Content of Research |
BTK_SIGNOFF_COMMENT |
|
The XSLT=”3500aForm.xsl” file is used to map the data fields from the complaint record to the MedWatch PDF form.
Application Name\SubmissionForm\FormConfig.xml
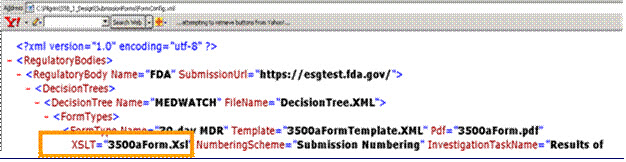
Example Scenario
The table below shows some fields that are normally mapped from the Complaint record into the HTML (3500aFormHtml.xsl) template or any PDF (3500aForm.xsl) report. The data fields that are available for mapping are located in the following views:
Field Label |
Data Source |
|
CustomerComplaint |
V_CXP_CUSTOMER_PXP
|
|
AdditionalInfo |
V_CEX_CXP_EXTENSION
|
|
SerialNumbers |
V_PXS_PXP_SERIAL
|
|
Attachments |
V_BAH_BASE_ATTACH_QXP
|
|
Contacts |
V_CMC_CXP_CONTACT
|
|
Part |
V_EPA_EXCEPTION_PART
|
|
Lots |
V_EPL_EXCEPTION_PART_LOT
|
|
Submissions |
V_BAH_BASE_ATTACH_CXP
|
|
GetInvestigationResult |
V_BTK_BASETASK
|
|
References Tab | External Reference |
Complaint/V_EXR_EXTERNAL_REFERENCE/EXR_REFERENCE_VALUE
|
|
NOTE: Each data field in the complaint record is associated with a specific view; basically use the prefix of the data field to find the appropriate view name to use in your expressions. For example, if you want to use a user defined field, CEX_UDF_STRING_2 and map it over you will need to use the view: V_CEX_CXP_EXTENSION |
· If the user does not want Complaint record data to flow into the follow-up reports, add an attribute called “NotInFollowUp”=”True”.
· If the user wants data to flow into Follow-Up reports set the True to False; for example, “NotInFollowUp”=”False”
Application Name\SubmissionForm\3500aForm.Xsl
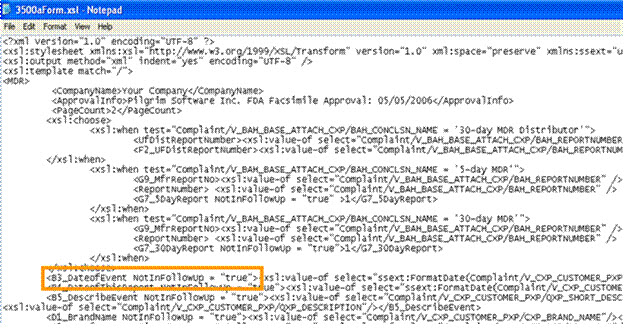
In the MedWatch PDF Report, the default Out of the Box PDF says Your Company.

The MedWatch PDF report needs to be changed to the company’s corporate name. This is setup once and will populate into all the MedWatch PDF files.
1. Access the folder - Application Name\SubmissionForms\3500aForm.xsl
2. Change Your Company to the name to be used for MedWatch reporting.